Abstract
Background: Increased evidence suggests the PD-1/PD-L1 axis is involved in the oncogenesis of myeloid neoplasms through promoting cancer immune escape, which suggests potential benefits by blockade of this axis in these neoplasms by immune checkpoint inhibitors. However, the ongoing clinical trials on PD-1/PD-L1 blocker in MDS/AML have reported controversial results, unlike the promising therapeutic efficacy shown in solid tumors. Identifying reliable biomarkers and scoring of PD-L1 expression and targeting the subgroup of patients who might benefit the most from PD-1/PD-L1 blockade are promising directions. Janus kinase 2 (JAK2) pathway activation has been shown to enhance PD-L1 expression in malignancies by in vitro studies. Here, we investigated PD-L1 expression associated with JAK2/STAT oncogenic pathway in AML patients using the combined positive score (CPS) algorithm.
Methods: Immunohistochemical staining (IHC) for PD-L1 and pSTAT3 was performed on the marrow biopsies of 31 AML patients (39 cases), including 11 patients (17 cases) with JAK2/STAT mutation and 20 patients (22 cases) with wild-type JAK2/STAT. For PD-L1 IHC, the cases were evaluated for variable staining intensity (weak, moderate, strong) and membrane staining pattern (partial, complete) in tumor and mononuclear immune cells. The PD-L1 CPS was calculated by summing the number of all PD-L1-stained cells and dividing by the total number of tumor cells, multiplied by 100. The pSTAT3 was evaluated by percentage of positive tumor cells among the total tumor cells. The status of JAK2 / STAT mutations and other molecular abnormalities was evaluated by next generation sequencing (NGS) study.
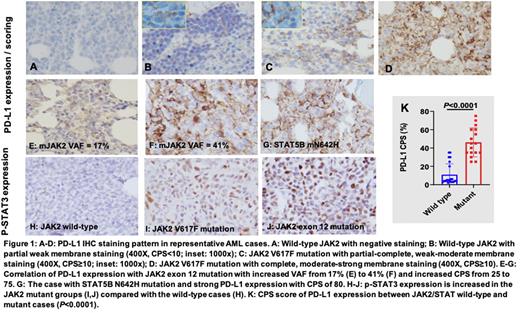
Results: Among patients of JAK2/STAT mutations, nine with JAK2 V617F exon 14 mutations, one with JAK2 I540-E543delinsRG exon 12 mutation and one with STAT5B N642H mutation. Evaluation of PD-L1 expression by IHC revealed four major cell staining patterns in our study cohort: 1. Negative (Figure 1A); 2. Partial weak membrane staining (Figure 1B, CPS<10); 3. Partial-complete, weak-moderate membrane staining (Figure 1C, CPS≥10); 4. Complete, moderate-strong membrane staining (Figure 1D, CPS≥10). Patterns 3-4 were classified as positive PD-L1 staining with a CPS cut-off of 10 (CPS≥10). Comparison of the PD-L1 CPS score in the JAK2/STAT wild-type versus the JAK2/STAT mutant groups revealed significantly higher PD-L1 CPS score in the JAK2/STAT mutant group (Figure 1K, P<0.0001, CPS≥10). One patient showed wild-type JAK2 at initial AML diagnosis. At two subsequent relapses, gain of JAK2 exon 12 (I540-E543delinsRG) mutation with increased VAF from 17% to 41% was detected. Interestingly, PD-L1 expression was correspondingly increased with CPS from 25 to 75 in these subsequent biopsies (Figure 1E, F). In another case with wild-type JAK2, STAT5B N642H mutation was detected and strong PD-L1 expression with CPS of 80 was obtained (Figure 1G). To address if JAK2/STAT3 axis is involved in PD-L1 upregulation, we assessed STAT3 phosphorylation by IHC in JAK2 wild-type and mutated groups. The results revealed significantly increased p-STAT3 expression in the JAK2 mutant compared with the wild-type cases, supporting the idea that oncogenic JAK2 mutation leads to STAT3 phosphorylation (Figure 1H-J). Furthermore, p-STAT3 expression showed a significant positive correlation with the PD-L1 CPS score (Figure 1H, r=0.8663, P<0.0001).
Conclusion: In the AML patients with oncogenic JAK2 or its downstream signaling molecule STAT5B mutations, there is significantly enhanced PD-L1 expression. In addition, we proposed that the PD-L1 CPS positivity cut-off score of 10 being used as a quantitative measure of PD-L1 expression in this heterogeneous myeloid neoplasm. In the patient with relapsing AML, PD-L1 expression showed a positive correlation with increased JAK2 mutant VAF. Independent of the oncogenic JAK2 mutation, PD-L1 expression upregulation was also noted with STAT5B N642H mutation. Lastly, we demonstrated enhanced p-STAT3 expression in JAK2 mutant cases and a positive correlation between p-STAT3 and PD-L1 expression. Taken together, these data support the model that JAK2/STAT pathway activation is associated with PD-L1 upregulation in AML patients (Figure 1I), together with the proposed PD-L1 CPS cut-off of 10, can serve as scanning biomarkers to select relevant subgroups of AML patients for PD-1/PD-L1 immunotherapeutic study.
Disclosures
Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:Calithera: Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Stemline therapuetics: Consultancy, Honoraria, Research Funding; Eli Lilly: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rafael Pharmaceutical: Research Funding; Sanofi: Research Funding; Forty Seven: Honoraria, Research Funding; AstraZeneca: Research Funding; Ascentage: Research Funding; Ablynx: Research Funding. Verma:BMS: Research Funding; Jannsen: Consultancy, Research Funding; Eli Lilly: Research Funding; Throws Exception: Current holder of stock options in a privately-held company; Bakx Therapeutics: Consultancy, Current equity holder in private company; Ionctura: Research Funding; Prelude: Research Funding; Novartis: Consultancy; Medpacto: Research Funding; Stelexis Therapeutics: Current equity holder in private company, Honoraria; Curis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal